Report Overview
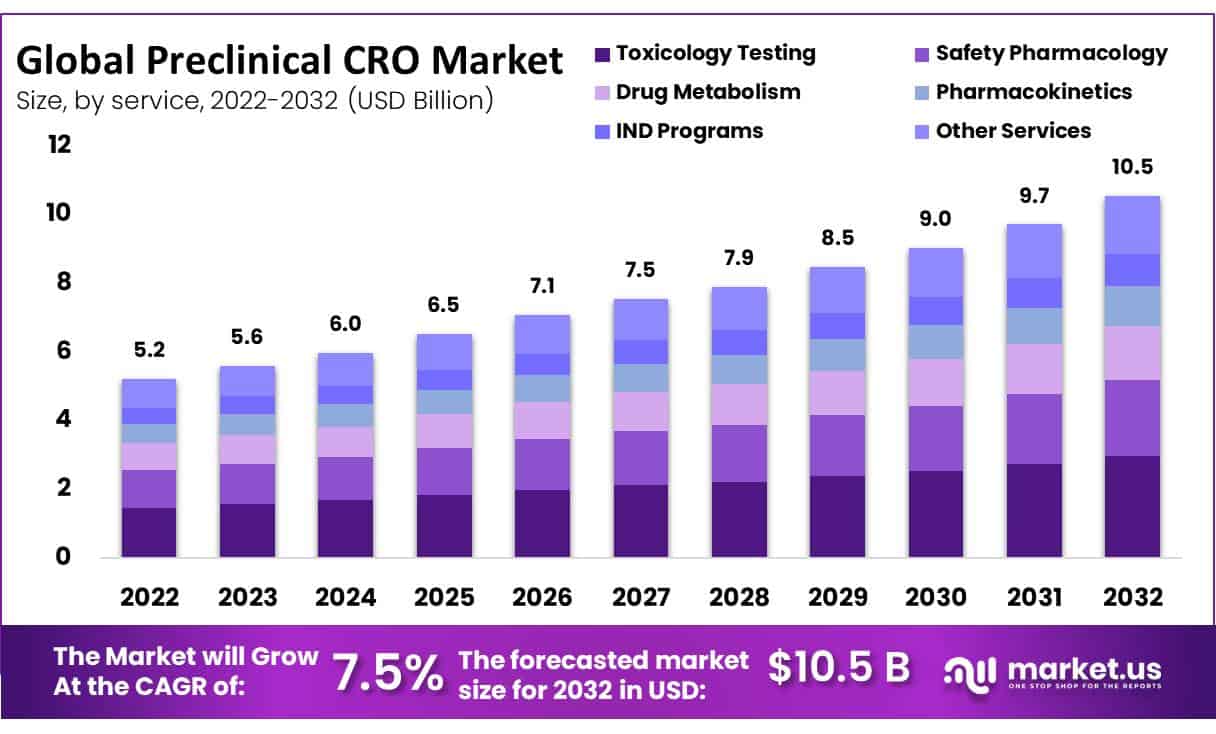
The Preclinical CRO Market size is expected to be worth around USD 10.5 Billion by 2032 from USD 5.2 Billion in 2022, growing at a CAGR of 7.5% during the forecast period from 2023 to 2032.
 Get a sample copy of the report https://market.us/report/preclinical-cro-market/request-sample/
Get a sample copy of the report https://market.us/report/preclinical-cro-market/request-sample/
Key Takeaways
In 2022, toxicology testing emerged as the highest revenue-generating service.
The Patient-Determined Organoid (PDO) Model segment dominated the market with an 80% share by Model Type.
Pharmaceutical and Biopharmaceutical Organizations were the major end-users in 2022.
North America retained its market leadership with a 47.50% share in 2022.
Asia Pacific is expected to experience significant growth with a substantial Compound Annual Growth Rate (CAGR) in the forecast period.
Key Market Segments
By Service
- Toxicology Testing
- Safety Pharmacology
- Drug Metabolism
- Pharmacokinetics
- IND Programs
- Other Services
By Model Type
- Patient Derived Organoid (PDO) Model
- Patient Derived Xenograft Model
By End-User
- Pharmaceutical and Biopharmaceutical companies
- Medical Device manufacturing companies
- Academic Research Organizations
- Other End Users
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Key Players
- PAREXEL International Corporation
- Laboratory Corporation of America Holdings
- Medpace, Inc.
- Envigo Corporation
- Charles River Labs
- PRA Health Science, Inc.
- PPD Inc.
- Covance Inc.
- Other Key Players.
If You Have Any Questions About This Report, Please Reach Out to Us
https://market.us/report/preclinical-cro-market/#inquiry
Drivers:
Increasing Outsourcing: Pharmaceutical firms are increasingly outsourcing their preclinical research activities to CROs to streamline operations and reduce costs.
Rising R&D Expenditure: Higher spending in the life sciences sector, particularly in drug development, is driving demand for preclinical CRO services.
Advancements in Technology: The adoption of advanced technologies such as in vitro assays, patient-derived models (PDOs), and AI-driven analytics is enhancing the efficiency and accuracy of preclinical studies.
Trends:
Shift towards Patient-Derived Models (PDOs): PDOs are gradually replacing animal models because of better predictive accuracy and better likeness to human physiology.
Demand for Specialized Toxicology Testing: The current trend toward the use of toxicology studies is aimed at the evaluation of safety and the compliance of new chemical compounds with stated regulatory requirements.
Opportunities:
Expansion in Emerging Markets: The growth signals that are expected in emerging markets such as Asia Pacific and Latin America is palpable because of the improving healthcare facilities and accelerated R&D investments.
Customized Preclinical Services: The current industry trend shows that Companies are looking forward to having a preclinical study done especially those that are specialized in dominant therapeutic areas and the breakdown of disease models may lead to several opportunities for CROs.
Restraints:
Stringent Regulatory Requirements: Rules and regulations of clinical research and a rigorous approval process stand out as the operational issues that affect ROCs since they prolong the time to market.
Cost and Pricing Pressures: The existing competition among CROs and bargaining with clients over the price of contracted services have a bearing on the CROs' profit and operational sustainability.
Contact Us :
420 Lexington Avenue, Suite 300 New York City, NY 10170,
United States
Phone:+1 718 618 4351 (International),+91 78878 22626 (Asia)
Email: inquiry@market.us